Beyond CO2
We have a holistic view of climate change and have addressed non-CO2 long-term greenhouse gases such as hydrofluorocarbons (HFCs), perfluorocarbons (PFCs), nitrous oxide (N2O) and sulfur hexafluoride (SF6). Through our Restricted Substance Management Standard we have prohibited SF6 in tires in magnesium casting. We are continuing our scientific research to determine the relative contribution of a wide range of long-lived greenhouse gases to radiative forcing of climate change.
In 2010, we worked with an international team of climate and atmospheric scientists under the auspices of the World Meteorological Organization to assess the global warming potentials of long-lived greenhouse gases. Given impressive reductions in the emission of criteria pollutants (hydrocarbons, NOx, particulate matter and carbon monoxide) enabled by improvements in engine and exhaust after-treatment technology, we believe that the contribution to climate change by these short-lived pollutants from light-duty vehicles will be of relatively minor importance in the future.1 We have presented a technical assessment arguing that time horizons of 20 years, or longer, are needed in assessments of the contribution of road transport to radiative forcing of climate change.2
While carbon dioxide is by far the most important greenhouse gas associated with the use of motor vehicles, small amounts of other greenhouse gases are also emitted, notably methane (CH4), N2O and hydrofluorocarbon-134a (HFC-134a). Methane is formed in the engine and emitted into the atmosphere. We have assessed the contribution to climate change made by methane emissions from vehicles as about 0.3 to 0.4 percent of that of the CO2 emissions from vehicles. We have assessed the contribution to climate change from N2O emissions from vehicle tailpipes (not including potential emissions associated with fuel production) as about 1 to 3 percent of that of the tailpipe CO2 emissions from vehicles. Finally, we have estimated that the radiative forcing contribution of HFC-134a leakage from an air-conditioner-equipped vehicle is approximately 3 to 5 percent of that of the CO2 emitted by the vehicle.3 When expressed in terms of “CO2 equivalents,” the contribution of vehicle emissions to radiative forcing of climate change is dominated by emissions of CO2.
CFCs, HFCs, HFOs and the Montreal Protocol
The Montreal Protocol on Substances that Deplete the Ozone Layer (1987) regulates the emissions of ozone-depleting substances such as chlorofluorocarbons (CFCs). Ford has been a leader in conducting research on CFC replacements. In 2010 we were awarded a U.S. Environmental Protection Agency Montreal Protocol Award in recognition of our work in this area. In the 1980s and early 1990s, all vehicle manufacturers used CFC-12 (CF2Cl2) as the refrigerant in air conditioning (AC) units. By the mid-1990s, vehicle manufacturers switched to hydrofluorocarbon-134a (also known as HFC-134a or CF3CFH2). Hydrofluorocarbons contain only hydrogen, fluorine and carbon. Hydrofluorocarbons do not contain chlorine and hence do not contribute to the well-established chlorine-based stratospheric ozone depletion chemistry. HFC-134a has a shorter atmospheric lifetime and smaller global warming potential than CFC-12 (see Table 1).
The lifecycle emissions of CFC-12 from AC-equipped vehicles in 1990 was approximately 400 g per vehicle per year.4 We estimate that lifecycle emissions of HFC-134a from vehicles manufactured in 2010 are approximately 100 g per vehicle per year.5 Looking to the future, based on published assessments,6 we believe that HFC-134a emissions from a typical light-duty vehicle manufactured in 2017 will be approximately 50 g per vehicle per year.
Regulations in the EU require us to use compounds with global warming potentials of 150 or less in the AC units of all new vehicles starting in 2011 and all registered vehicles starting in 2017. HFC-134a has a global warming potential of 1,370,7 and the automotive industry will not be able to use this compound in the future in new vehicles in the EU. Hydrofluoroolefins (HFOs) are a class of compounds that are safe for the ozone layer and have very small global warming potential (typically <10). Based upon engineering, environmental and safety assessments, Ford has chosen the compound known as HFO-1234yf (also known as HFC-1234yf or CF3CF=CH2) for use in our European vehicles subject to the above-mentioned legislation timing. Research at Ford8 has established that HFO-1234yf has a global warming potential of 4.
To place the emissions of CFC-12, HFC-134a and HFO-1234yf into perspective, we can compare their contribution to radiative forcing of climate change with that of CO2 emitted by the tailpipe of the vehicle. Figure 1 shows this comparison for a typical car in the U.S. from 1990, 2010 and 2016. The CO2 equivalent (CO2eq) contributions from refrigerants in Figure 1 were calculated assuming a CFC-12 AC system in 1990, an HFC-134a system in 2010 and either an HFC-134a or an HFO-1234yf system in 2016. The CO2eq values for CFC-12, HFC-134a and HFO-1234yf were calculated using the emission estimates given above and the global warming potentials given in Table 1. The tailpipe CO2 values were calculated using the U.S. National Highway Traffic Safety Administration requirement fuel economies of 27.5 mpg in 1990 and 2010 and 37.8 mpg in 2016 and assuming the car is driven 10,000 miles per year.
As seen in Figure 1, the emissions of CFC-12 from an AC-equipped car in 1990 had a climate impact that was actually greater than that of the CO2 emitted from the tailpipe of the car. Replacement of CFC-12 with HFC-134a, together with improvements in the AC system, has led to a dramatic (approximately 30-fold) decrease in the climate impact of refrigerant emissions per vehicle for an AC-equipped vehicle (compare the two left-hand columns in Figure 1). Looking to the future, we anticipate a further – approximately factor of two – decrease in the impact of HFC-134a emissions on a per-vehicle basis (see the third column in Figure 1). Replacing HFC-134a with HFO-1234yf leads to a further decrease in the climate impact, and the AC refrigerant impact ceases to be discernible in the right-hand column in the figure.
The U.S. Environmental Protection Agency has proposed that HFCs such as HFC-134a should be added to, and regulated as part of, the Montreal Protocol. We do not support the inclusion of HFCs within the Montreal Protocol based upon three well-established scientific facts:
First, HFCs do not contribute to the depletion of stratospheric ozone. HFCs should therefore not be included in the Montreal Protocol on Substances that Deplete the Ozone Layer.
Second, as seen in Figure 1, replacing CFC-12 by HFC-134a has been a major step forward in environmental protection. Retaining the option to use HFC-134a in the future increases our ability to deliver cost-effective solutions for our customers.
Third, emissions of CO2, CH4 and N2O, not HFCs, are the main driver of climate change. (HFCs are currently responsible for less than 1 percent of the radiative forcing by long-lived GHGs.) Regulations focused on less than 1 percent of the problem are not very useful. We need to adopt a lifecycle perspective and focus on the most cost-effective options. More study, including an assessment of cost effectiveness, is required before enacting blanket restrictions on HFCs.
Figure 1: Annual in-use greenhouse gas (GHG) emissions from typical AC-equipped cars in the U.S in 1900, 2010 and 2016 using either CFC-12 (in 1990, left-hand bar), HFC-134a (2010 and 2016, middle bars), or HFO-1234yf (right-hand bar) refrigerants.
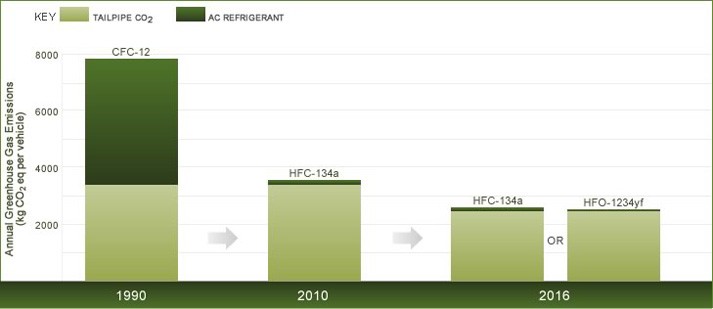
Table 1: Comparison of CFC-12, HFC-134a and HFO-1234yf
| Compound | Chemical Formula | Safe for Ozone? | Atmospheric Lifetime9 | Global Warming Potential9 |
|---|---|---|---|---|
| CFC-12 | CF2CI2 | No | 100 years | 10,900 |
| HFC-134a | CF3CFH2 | Yes | 13.4 years | 1,370 |
| HFO-1234yf | CF3CF=CH2 | Yes | 11 days | 4 |
- T.J. Wallington, J.E. Anderson, S.A. Mueller, S. Winkler and J.M. Ginder, “Emissions Omissions,” Science 327, 268, (2010).
- T.J. Wallington, J.E. Anderson, S.A. Mueller, S. Winkler, J.M. Ginder and O.J. Nielsen, “Time Horizons for Transport Climate Impact Assessments,” Environ. Sci. Technol. 45, 3169 (2011).
- T.J. Wallington, J.L. Sullivan and M.D. Hurley, “Emissions of CO2, CO, NOx, HC, PM, HFC-134a, N2O and CH4 from the Global Light Duty Vehicle Fleet,“ Meteorol. Z. 17, 109 (2008).
- IPCC/TEAP, Special Report: Safeguarding the Ozone Layer and the Climate System, Cambridge University Press, 2005.
- T.J. Wallington, J.L. Sullivan and M.D. Hurley, “Emissions of CO2, CO, NOx, HC, PM, HFC-134a, N2O and CH4 from the Global Light Duty Vehicle Fleet,” Meteorol. Z. 17, 109 (2008).
- S. Papasavva, D.J. Luecken, R.L. Waterland, K.N. Taddonio and S.O. Andersen, “Estimated 2017 Refrigerant Emissions of 2,3,3,3-tetrafluoropropene (HFC-1234yf) in the United States Resulting from Automobile Air Conditioning,” Environ. Sci. Technol. 43, 9252 (2009).
- World Meteorological Organization, Scientific Assessment of Ozone Depletion: 2010, Geneva (2010).
- O.J. Nielsen, M.S. Javadi, M.P. Sulbaek Andersen, M.D. Hurley, T.J. Wallington and R. Singh, “Atmospheric Chemistry of CF3CF=CH2: Kinetics and Mechanisms of Gas-Phase Reactions with Cl Atoms, OH radicals, and O3,” Chem. Phys. Lett. 439, 18 (2007).
- Data source: WMO/UNEP, Scientific Assessment of Ozone Depletion: 2010, Geneva (2010). Global Warming Potential is a relative measure of how much heat a greenhouse gas traps in the atmosphere. It compares the amount of heat trapped by a certain mass of the gas in question to the amount of heat trapped by a similar mass of carbon dioxide. A GWP is calculated over a specific time interval, commonly 20, 100 or 500 years. GWP is expressed as a factor of carbon dioxide (whose GWP is standardized to 1).
